Research Article
Pesticide Use in Rice with Emphasis on BPH and Residue Implications in Rice Grain Trade 
2 Plot-85, Road-3, House No. 3-8-244/1, Chandrapuricolony, L.B. Nagar, Hyderabad-500074 (Telangana State), India
 Author
Author  Correspondence author
Correspondence author
Molecular Entomology, 2018, Vol. 9, No. 2 doi: 10.5376/me.2018.09.0002
Received: 02 Mar., 2018 Accepted: 28 May, 2018 Published: 15 Jun., 2018
Krishnaiah N.V., 2018, Pesticide use in rice with emphasis on BPH and residue implications in rice grain trade, Molecular Entomology, 9(2): 11-28 (doi: 10.5376/me.2018.09.0002)
When BPH attacks rice crop during flowering and grain hardening stage, it necessitates a pesticide application. That application(s) is likely to lead pesticide residues in grain. Such residue can be harmful to the health of the consumer or can become a cause of rejection of the grain when exported to foreign countries. Hence it is essential to have understanding about the residues of various pesticides recommended against BPH (and/or other pests) with regard to their residue implications. This paper describes briefly the analytical procedures for some important insecticides recommended and used against BPH like buprofezin, ethofenprox, thiamethoxam, chlorantraniliprole, Imidacloprid, pymetrozine, cyantraniliprole, fipronil and denotefuran, in different rice growing countries with special reference to their implications in international rice trade. However, it is painful to admit that there are no analytical methods available in literature for ethiprole and sulfoxaflor residue analysis in rice grain.
Background
The term pesticide in its broadest meaning is any substance that is used to kill the pest. When we consider the broadest meaning of the term pest, it includes “any organism that competes with humans for food, space other resources or damage his possessions or cause direct or indirect harm to humans.” From the point of agriculture, the insects, mites, pathogens, weeds, rodents, some snails, nematodes or sometimes even birds are considered to explain the term “pests”. Hence insecticides, acaricides, fungicides, herbicides, rodenticides, nematicides all come under the broad term of “pesticides”. However, Environmental protection agency of U.S. has included other materials like plant growth regulators, defoliants, desiccants or nitrogen stabilizers also under the term pesticide and defined as “A pesticide is any substance or mixture of substances intended for Preventing, destroying, repelling or mitigating any pest, Use as a plant regulator, defoliant, or desiccant, or used as a nitrogen stabilizer.” (US EPA, 2010)
Before we go into the details about pesticides let us first explain what a pest really means. When we say that “any organism that competes with humans” the term “pest” itself is subjective and certainly not an objective term. It means that humans have come into existence through evolution and to be frankly speaking all other organisms also came in to existence through the same phenomenon of evolution. That means every organism on the earth has equal right for its own existence, survival and perpetuation. Whatever may be the cause of evolution and origin of evolution; all organisms are equal in the eyes of that entity what many people describe as “GOD” or in any other name. In this context it is worth recollecting some of the ideas and thoughts propounded by Swami Vivekananda. He argues “Suppose if a human being thinks that God could all probability be existing in human form, then what a buffalo should think. Buffalo could also be thinking that God should be in its own form or for that matter every living organism by all probability be thinking that God exists in its own form.” When we consider the whole of evolution the origin and cause of the whole existence, then all organisms are equal in the eyes of God or for that matter whatever one may call that entity of eternity in time, space and causation. If humans extend their thinking up to that level, then the term pest and its meaning as explained above is certainly one sided and partial. The very fact that humans acquired a better cerebrum to think and act than all other organisms does not really sanctify that any organism that acts against humans should not exist in nature. So pests also exist and will be existing as long as there is existence.
1 Organic Cultivation vs. Pesticide Application
Now let us come back to explain about the importance of pesticides. Many scientific philosophers consider that pesticides are necessary evils which have to be used to safeguard the humans from extinction. Another important point one has to think about pesticides is even if any crop of agriculture or horticulture is intended to be grown purely organically without using any chemical fertilizer then also there is no guarantee that “that particular crop” will not be damaged by an insect or a pathogen or any other pest. When an insect like BPH attacks the crop and starts damaging, farmer cannot afford to keep quiet without applying a suitable pesticide say monocrotophos, or acephate or buprofezin or dinotefuran. When once a pesticide is applied to the crop that crop will no more be valid for consideration as a purely organically grown crop.
Another important point to be considered is when a crop like rice is intended to be grown organically the yield of that crop is bound to be far lower than a side by field which is grown with fertilizers and pesticides. There are certain basic differences among various countries regarding the term organically grown crop. In U.S., Canada, Australia and some European countries which are technologically more advanced allow limited fertilizer use and use of the most essentially applied pesticides like herbicides as admissible under organic cultivation. However, the crop needs to be applied with sufficient organic matter to meet all its nutritional requirements with regard to all nutrients except probably N, P and K. Contrary in countries like China and India which are mostly tropical with more pest prone conditions, in the case of organically grown crop “no fertilizer, no herbicide or no other pesticide application” is the rule. If we critically see the entire rice growing tracts in India there is no place where a farmer can grow rice without using a pesticide. To the author’s best judgment and that of many other scientists’ only Kashmir valley with approximately 148,000 hectares which is equivalent to 0.33% of the total area of 45 million hectares in India is suitable for organic rice cultivation. In any other area even if farmer attempts to grow rice organically with any of the varieties he cannot harvest rice crop even at minimum yield level that is possible with pure organic manures. He needs to give at least one or two applications of an insecticide or a fungicide. This also is possible only when he can afford to lose rice crop without herbicide or can do weeding manually. With the existing conditions in demand and cost of labor it is impossible for any rice farmer to execute manual weeding. Almost similar situation could be existing in many south Asian and South-East Asian countries which are major tropical rice belts.
2 Pesticide Residues in Theory and Practice
Having considered what a pest is and pesticide is, let us now focus on what a pesticide residue means. When any pesticide is applied to rice crop the entire quantity will not get degraded in soil, water or crop or dissipated in to the air. Let us consider the theoretical aspect of it. It is well known that a gram molecular weight of any substance contains approximately 6.25 x 1024 molecules which is Avogadro’s Constant or Avogadro’s number. So even if a few grams of a pesticide are applied to rice crop it can have approximately 1022 to 1023 molecules. By utilizing the best possible analytical technology for any pesticide available even today, it is not possible to detect below picogram level (10-12). Still approximately 109 to 1010 number of molecules of that pesticide can be present in soil, water or crop. Therefore a large number of molecules of any pesticide can be present in soil or water or rice grain or rice straw without being detected by humans.
3 Agencies Responsible for Fixing Pesticide Tolerance Limits in Rice Grain in Different Countries
The responsibility of fixing pesticide tolerance limits in different countries lies with different agencies. For instance in U.S., Environmental Protection Agency (EPA) takes the major responsibility while other agencies like United States Department of Agriculture (USDA), and Food and Drug Administration (FDA) assist EPA. These are called “tolerance limits” in U.S. and maximum residue limits (MRLs) in other countries.
In India, The Department of Agriculture, Cooperation & Farmers Welfare, under Ministry of Agriculture & Farmers Welfare has a central sector scheme, “Monitoring of Pesticide Residues at National Level” in food commodities and environmental samples with the participation of various laboratories representing Ministry of Agriculture, Indian Council of Agriculture Research, Ministry of Health and Family Welfare, Ministry of Environment and Forests, Council of Scientific and Industrial Research, Ministry of Chemicals and Fertilizers, Ministry of Commerce and State Agricultural Universities across the country. Food Safety and Standards Authority of India, Ministry of Health and Family Welfare Government of India, New Delhi, is the executive authority for fixing the maximum residue limits (MRLs) for pesticides in different food items including rice grain in India.
Health Canada is the organization that is concerned with pesticide residues in agricultural commodities produced locally as well as those which are imported. In Brazil Anvisa is the agency concerned with pesticide residues. In Europe, The European Food Safety Authority (EFSA) fixes and assesses the maximum residue limits (MRLs) of pesticides expected on food and different diets of Europeans and ensures safety for consumers. In Australia as well as in New Zealand, MRLs of pesticide residues is under the jurisdiction of Food Standards Code of Australia and New Zealand.
In Korea, Korea Food and Drug Administration (KFDA) along with Rural Development Administration (RDA) executes the responsibility of fixing pesticide residues in food items.
In Japan, Pesticide Residue Analysis Research Group (PRARG), Tokyo is responsible for fixing maximum residue limits (MRLs) in different food items including rice grain.
At international level, “C O D E X A L I M E N T A R I U S” established by Food and Agricultural Organization (FAO) and the World Health Organization (WHO) in 1963 is responsible to develop harmonized international food standards, guidelines and codes of practice. These contribute to the safety, quality and fairness of international food trade. Consumers can trust the safety and quality of the food products they buy and importers can trust that the food they ordered will be in accordance with their specifications. For execution of this, Joint FAO/WHO Meeting on Pesticide Residues (JMPR) of expert committee is held periodically to discuss and finalize the scientific aspects of pesticide tolerances in rice grain along with other food items.
4 Brief History of Pesticide Residue Analysis
The field of pesticide residue analysis came into existence only after second world-war. That is also the period when major developments took place in pesticide synthesis, production and use. Similar to the development of pesticide technology the starting point for pesticide residue analysis is also DDT, HCH and the first generation organophosphate and methyl carbamates among insecticides and organo-mercurial and dithiocarbamates among fungicides, 2, 4-D and other related molecules among herbicides. The recognition of bio-magnification of DDT and HCH at higher trophic levels that led to extinction of some birds and other animals was actually possible after the invention of gas liquid chromatography with different detectors. Electron capture detector was widely employed for halogen containing compounds like DDT, HCH and 2, 4-D etc., while modified flame ionization detector was popular for most organophosphates. However, for methyl carbamates and dithiocarbamates colorimetric techniques were originally used which were later replaced by derivatization technology. With the development of mass-spectra attached either to gas chromatography (GC) or to high pressure liquid chromatography (HPLC) the pesticide analysis has really made rapid strides along with the whole of the analytical field. Further, during nineteen nineties synthetic pyrethroids, neonicotinoids, insect growth regulators like buprofezin, ether derivatives like ethofenprox etc. came into prominence. All these groups of pesticides are biodegradable and also recommended at low dosages. Thus these are likely to leave lower level of residues in the produce. Among fungicides and herbicides also, latest groups of chemicals are used at lower dosages, biodegradable and possess lower toxicity to higher animals including humans. All these developments actually reduced the importance of pesticide residues in agricultural produce during recent times.
Among different countries that took lead in development of technology on pesticide residue analysis is also U.S. followed by some European countries like U.K. Germany, France etc and Japan among Asian nations. Later the developing countries like India, China, many South Asian and South-East Asian countries like Indonesia, Malaysia, Thailand, Vietnam, and Philippines etc. followed the line. Food and Agricultural Organization (FAO) and World Health Organization (WHO) coordinated the whole research efforts on pesticide residues as the subject concerns both these international organizations. It may not be out of place to mention that in countries like India where there was large scale imports of food grains under PL480 during 1950 s from USA resulted in accumulation of money domestically. Those funds were utilized for improving the facilities for pesticide residue analysis and enhancing the technical knowledge in many agricultural universities. The author is very happy to inform that his Ph.D. problem under the guidance of Dr. R.L. Kalra was a part of the technical program of PL480 project on pesticide residues at Punjab Agricultural University, Ludhiana, Punjab, India.
5 Necessity of Having Pesticide Residue Analysis in Rice Grain
Almost all pesticides are toxic to humans or other higher or lower animals to a lesser or higher degree. Therefore the residues of these pesticides are also bound to be toxic to humans as well as others in animal kingdom. Herbicide residues can exert harm to non-target plant species and some other crops. Hence every effort must be exerted to minimize the toxicity of pesticide residues. To achieve this purpose, the tolerance limit for each pesticide in a given produce say paddy grain must be fixed. Further legal provisions must also be made to impose these “pesticide tolerance limits” in every produce consumed by humans and domestic animals. So pesticide tolerance limit in any agricultural produce or most importantly in rice grain is a limit fixed by legal authority and if the residue of that pesticide is present in an agricultural produce above that particular limit then the produce will not be allowed in the market.
Rice is a major food crop for the people of the world in general and Asians in particular. Nearly 90% of the world’s rice is produced and consumed in Asia. Furthermore, rice is a staple food for nearly 2.4 billion people in Asia. Except for Pakistan and some parts of India and China, rice provides two thirds of the calories for most Asians with rice-based diets. This speaks volumes about the importance of rice grain to be free from toxic contaminants originating from pesticides and strict regulations to be imposed to see that rice imports contain pesticide residues below maximum residue limits (MRLs).
BPH attacks rice crop at all growth stages from nursery to harvest. However, its damage is typically severe from panicle initiation up to harvest. Hence it is essential to apply recommended insecticides to rice crop even a few days before harvest. This is likely to leave harmful pesticide residues in the grain at harvest time. Therefore, it is necessary to have information on residue implications of each of the recommended insecticides against BPH in harvested paddy as well as straw.
Stem borers and gundhi bug are other insect pests that can damage rice crop during flowering stage and many a times demand suitable and recommended insecticide application. When a single insect attacks the crop one or two applications of recommended insecticide(s) against the target pests are sufficient. The most important point to be kept in mind is that the spectrum of effective insecticides against BPH is much different from those which are effective against stem borer. Hence many a times it is essential to use combinations of insecticides to ward off the crop from both stem borer, BPH and if necessary even gundhi bug. Further, when slow acting insecticides like buprofezin or fipronil or ethiprole are applied against BPH it is the normal practice to use quick acting insecticides like neonicotinoids or diclorvos in combination. Hence it is important to consolidate the available information on residue analysis of the most important insecticide molecules recommended and used against BPH and stem borer and likely to leave residues in rice grain and analyze the information critically.
6 Normal Procedure for Fixing Tolerance Limit (TL) or Maximum Residue Limit (MRL)
For a given pesticide first of all acceptable daily intake (ADI) is determined through experimentation with mice or rabbits. ADI is the dose of a particular pesticide which is not likely to cause any harm to any system of humans even if it is consumed daily throughout life based on the knowledge available at that time. Since it is humanly impossible to directly experiment the chemical on human beings, experiments are first designed to be conducted on mice or rabbits for ease and convenience and the effect of the chemical on different organs are observed through short term and long term experimentation. Physiological observations on vital systems like digestive, respiratory, circulatory as well as histological observations on all tissues is assessed in the short range and long range studies. Finally the dose of the chemical at which there are no adverse health effects of the organism is determined. After that a ten times allowance is given to take care of the difference between species and ten times allowance is given for difference among human individuals. So the dose of the pesticide which has been arrived at as no effect level for the animals is divided by a factor of hundred and that is fixed as acceptable daily intake for humans.
After that, experiments are conducted with the chemical on a given crop say rice in our case and actual residues that will remain in grain and straw are determined by following a good agricultural practice as recommended by the research organization or a university. The normal residues of the chemical on rice grain and straw are determined. If the residues of a pesticide that remain in rice grain by following a good agricultural practice is less than the one arrived through acceptable daily intake approach, then MRL is fixed as the value prevailing by following good agricultural practice.
In general, risk assessment is developed for each crop use of a pesticide that considers: The aggregate, non-occupational exposure from that pesticide i.e. exposure through diet, drinking water and from pesticides used in and around the home etc. Further, the cumulative effects from exposure to pesticides that have a common biochemical mode of action are assessed. It is also taken into consideration, whether there is increased susceptibility to infants and children or other sensitive subpopulations, from exposure to the pesticide. Endocrine disruption-effects i.e. whether the pesticide produces an effect in people similar to an effect produced by a naturally occurring estrogen is also taken into consideration.
7 Methodology for Determination of Pesticide Residues in Rice Grain
7.1 General guide lines for initial preparation of materials involved in pesticide residue analysis of rice grain
Analytical standard of the insecticide can be obtained from the manufacturing firm of that particular insecticide. Standard stock solution of approximately 500 mg/L can be prepared in suitable solvent and stored in refrigerator. It is required that the stock solution should be stable at 4°C for at least 6 months. Working solutions of insecticides, either for fortification or for HPLC or GLC calibration can be freshly prepared in suitable solvents or solvent mixtures depending on the requirement. The solid materials used for column chromatography like Florisil or silica gel (60~100 mesh) can be purchased from a standard company. Florisil needs to be activated at 130°C for more than 5 h prior to use. Solvents like acetonitrile, and deionized water used in HPLC must be HPLC grade. Generally, solvents used for extraction and clean up like acetonitrile; acetone, n-hexane etc. can be pesticide residue grade or reagent grade and need to be freshly redistilled in glass. Hulled rice of a locally available variety can be procured from an organically-grown field where no pesticides had been applied. Composite rice samples can be prepared in compliance with the international Guidelines for Pesticide Persistence (US FDA, 1999). Rice grains can be finely pulverized to pass through 40-mesh sieve and stored frozen at -20°C until analysis.
7.2 Buprofezin residue analysis in rice grain (Adopted from Lee and Jang, 2010)
Buprofezin [(Z)-2-tert-butylimino-3-isopropyl-5-phenyl-1, 3, 5-thiadiazinan-4-one] is a persistent insecticide which has high degree of efficacy against BPH. Buprofezin is a contact and stomach poison which is not translocated in the plant. The insecticide kills nymphs at the time of moulting and also drastically lowers the reproductive potential of the exposed adults. The detailed physiological mode of action of buprofezin against BPH has been dealt elsewhere (Krishnaiah, 2017). By following a good agricultural practice the normal level of residues of buprofezin in brown rice is expected to be around 0.05~1.0 mg/kg (US FDA, 1999). Hence it requires a very sensitive method of detection and analysis with lowest detectability limit of 0.02 mg/kg or less to fulfill the Guideline on Method of Pesticide Residue Analysis (CAC, 2003; RDA, 2004).
7.2.1 Need for developing residue analysis method for buprofezin by using HPLC
Buprofezin exhibits high lipophilicity of log Pow (n-octanol-water partition coefficient) 4.9 and vapor pressure of 4.2 × 10-2 mPa (20°C) (Tomlin, 2009). Gas-liquid chromatography (GLC) with nitrogen-phosphorus detector (NPD) or flame-photometric detector (FPD) seems to be preferable to analyze the residues at MRL level. However, thiadiazine ring and imino group in the molecule may cause adsorption or heat-lability which leads to peak asymmetry and poor reproducibility (Lee and Kwon, 2004). As vapor pressure of buprofezin is relatively low, high column temperature is quite necessary to elute the compound for the practical time of analysis. In this case, the undesirable effect would be more severe. Furthermore, consistent quantitation may not be guaranteed because the stability of NPD is known to be just fair and large fluctuation of response is frequently found being entirely dependent on alkaline bead condition (US FDA, 1999). FPD in sulfur mode is presumed to be insufficient sensitivity as buprofezin possesses only one sulfur atom. Considering these drawbacks, GLC is inadequate for the precise determination of buprofezin residues for the official purpose. Therefore an alternative method using HPLC needs to be developed to ensure high reproducibility along with enhanced sensitivity.
7.2.2 Extraction and partition for buprofezin residues
To a 25 g of rice sample in a 500-mL homogenizer cup 20 mL of distilled water can be added to moisten the sample. After brief shaking and standing for 30 min, 100 mL of acetone is added. The mixture can then be macerated at 10,000 rpm for 2 min in a high-speed homogenizer. The homogenate can be suction-filtered through the filter paper on porcelain Büchner funnel. The cup and filter cake is washed with fresh 50 mL of acetone, and the rinsate is combined with the previous filtrate. The filtrate can be quantitatively transferred to a one Litre separatory funnel. This can be followed by sequential addition of 100 mL of n-hexane, 50 mL of saturated sodium chloride solution, and 450 mL of distilled water to the one Litre separatory funnel. After vigorous shaking for 1 min and standing until two layers clearly separated, the lower aqueous phase is discarded. The hexane phase can be dried over 20 g of anhydrous sodium sulfate layer, collected in a 250-mL distilling flask. The hexane can then be evaporated just to dryness in vacuo at 40°C. The residue is dissolved in 40 mL of n-hexane saturated with acetonitrile and transferred to a 250-mL separatory funnel. The hexane phase can then be extracted twice with 30 mL portions of acetonitrile saturated with n-hexane. The acetonitrile extract is combined in a 125-mL distilling flask, and evaporated just to dryness in vacuo at 40°C. The residue can be dissolved in 10 mL of n-hexane and subjected to Florisil column chromatography (Lee and Jang, 2010).
7.2.3 Florisil column chromatography for buprofezin residues
A chromatographic column (11 mm i.d. × 40 cm) is plugged with glass wool, dry packed with 5 g of activated Florisil, and topped with approximately 2 cm layer of anhydrous sodium sulfate. The column can be pre-washed by passing 25 mL of n-hexane until the solvent level reaches the top of the sodium sulfate layer. A 5 mL aliquot of the hexane extract from the partition step is poured into the column and the column wall is rinsed twice with 2 mL portions of n-hexane. When the liquid drained to sodium sulfate layer, the column is eluted with 50 mL of dichloromethane/acetonitrile/n-hexane mixture (50/0.5/49.5, v/v/v), and the fraction is discarded. The column is then eluted with 50 mL of dichloromethane/acetonitrile/n-hexane mixture (50/1.5/48.5, v/v/v). The eluate can be collected, rotary-evaporated just to dryness at 40°C. The residue can be reconstituted with 5 mL of acetonitrile/water mixture (70/30, v/v) for HPLC determination.
7.2.4 High performance liquid chromatography (HPLC) for buprofezin residue quantitation
High-performance liquid chromatography (HPLC) of Lee and Jang (2010) consisted of Waters (USA) 515 pumps, 680 gradient controller, 2,489 UV/VIS absorbance detector, Agilent (USA) 1,100 auto-sampler and column oven, and Younglin (Korea) Autochro 2,000® data module/processing software. Hypersil Gold® C18 (4.0 mm i.d. × 250 mm, 5 μm spherical, Thermo, USA was used as the analytical column. Operating parameters used for the determination of buprofezin residues are; column temperature 40°C; mobile phase, acetonitrile/water (70/30, v/v), isocratic; flow rate, 1.0 mL/min; detection, UV absorption at 250 nm, 0.008 AUFS; sample size, 20 μL. Under these conditions, retention time of buprofezin is 9.1 min.
7.2.5 Validation of the analytical method and confirmation of the buprofezin residue
To validate the analytical method for buprofezin residues, series of control samples can be fortified with buprofezin standard solution in acetonitrile at different concentrations prior to extraction. After standing for 2 hours, analytical procedures mentioned above can be performed to assess the percentage recoveries from fortified samples.
LC/MS can be used to confirm the buprofezin residue in rice grain extracts. Buprofezin can also be separated from sample co-extractives on octadecylsilyl columns containing the packing material identical to HPLC determination. Operating parameters of LC/MS can be carefully optimized for maximum ionization of buprofezin.
7.3 Ethofenprox residue analysis in rice grain (Adopted from Lee et al., 2011)
Ethofenprox [2-(4-ethoxyphenyl)-2-methylpropyl 3-phenoxybenzyl ether] is an insecticide similar to synthetic pyrethroids but unlike most synthetic pyrethroids which are esters, ethofenprox is ether derivative. Ethofenprox acts by disturbing the neural function of BPH by interaction with sodium channel (Casida and Durkin, 2013). It is a contact and stomach poison and one of the effective insecticides recommended for BPH management in rice. In addition ethofenprox exhibits moderate efficacy against other insect pests on rice like leaf folder and stem borer also. Ethofenprox has very low toxicity to mammals, fish and other aquatic organisms which enables its use on rice. As one of the insecticides recommended for BPH management and its likely use during flowering and grain hardening stages of rice, there is need to assess terminal residues of ethofenprox in rice grain. Consequently, development of a reliable analytical method is prerequisite for estimation of the residue in rice grain. As maximum residue limits (MRLs) of ethofenprox in rice grain is 0.05 mg/kg (KFDA, 2009), highly sensitive method is required to meet 0.02 mg/kg of limit of quantitation (LOQ) or less to fulfill guidelines on analytical method for pesticide residues (CAC, 2003; RDA, 2004).
7.3.1 Necessity for developing residue analysis method for ethofenprox by using HPLC
Similar to most of synthetic pyrethroids, ethofenprox is a nonpolar molecule with thermal stability and volatility enough to be analyzed by gas-liquid chromatography (GLC). But in most of the synthetic pyrethroid molecules, the residues have been usually determined by electron-capture detection (ECD) at high sensitivity by taking advantage of halogen or nitrile group present. However, ethofenprox is unfortunately a simple hydrocarbon molecule with no atom or moiety sensitive to any specific detectors commonly used in GLC. Therefore, ethofenprox has not been included as an analyte in KFDA and US FDA official multi-residue analytical method in rice grain (KFDA, 2009; US FDA, 1999). Hence, it is essential to have a new method to analyze intact ethofenprox residues in rice grain samples using high-performance liquid chromatography (HPLC) with higher reliability and sensitivity, for the official analysis.
7.3.2 Extraction and partition for ethofenprox residues in rice grain
To a 25 g of rice in a 500-mL homogenizer cup, 20 mL of distilled water can be added to moisten the sample. After brief shaking and standing for 30 min, 100 mL of acetone can be added. The mixture is then macerated at 10,000 rpm for 2 min in a high-speed homogenizer. The homogenate can be suction-filtered through the filter paper on porcelain Büchner funnel. The cup and filter cake are washed with fresh 50 mL of acetone, and the rinsate is combined with the previous filtrate. The filtrate can be quantitatively transferred to a 1-Litre separator funnel, and sequential addition of 100 mL of n-hexane, 50 mL of saturated sodium chloride solution, and 450 mL of distilled water can be followed. After vigorous shaking for 2 min and standing until two layers clearly separated, the lower aqueous phase can be discarded. The hexane phase is dried over 20 g of anhydrous sodium sulfate layer, collected in a 250-mL distilling flask, and evaporated just to dryness in vacuo at 40°C. Then the residue can be dissolved in 40 mL of n-hexane saturated with acetonitrile and transferred to a 250-mL separator funnel. The hexane phase is then extracted three times with 30 mL portions of acetonitrile saturated with n-hexane. The acetonitrile extract can be combined in a 125-mL distilling flask, and evaporated just to dryness in vacuo at 40°C. The residue can be dissolved in 10 mL of n-hexane and subjected to Florisil column chromatography.
7.3.3 Florisil column chromatography
A chromatographic column (11 mm i.d. × 40 cm) can be plugged with glass wool, dry packed with 5 g of activated Florisil, and topped with approximately 2 cm layer of anhydrous sodium sulfate. The column can be pre-washed by passing 25 mL of n-hexane until the solvent level reached the top of sodium sulfate layer. The hexane extract (10 mL) from the partition step can be poured into the column and the column wall is rinsed twice with 2 mL portions of n-hexane. When the liquid drained to the sodium sulfate layer, the column can be eluted with 50 mL of dichloromethane/n–hexane mixture (70/30, v/v), and the fraction is discarded. The column can be then eluted with 60 mL of dichloromethane. The eluate is collected, rotary-evaporated just to dryness at 40°C, and the residue can be reconstituted with 10 mL of acetonitrile for HPLC determination.
7.3.4 High-performance liquid chromatography (HPLC) for ethofenprox residues in rice
High-performance liquid chromatography (HPLC) of Lee et al (2011) consisted of Jasco (Japan) 2,080 Plus pumps with built-in gradient former, 1,575 UV/VIS absorbance detector, 2,055 S autosampler, and Younglin (Korea) CTS30 column oven and Autochro 2,000® data module/processing software. Purospher STAR® RP18e (4.0 mm i.d. × 250 mm, 5 μm spherical, Merck, Germany) is used as the analytical column. Operating parameters used for the determination of ethofenprox residues are as follows; column temperature 40°C; mobile phase, acetonitrile/water (85/15, v/v), isocratic; flow rate, 0.8 mL/min; detection, UV absorption at 225 nm, 0.008 AUFS; sample size, 20 μL. Under these conditions, retention time of etofenprox is 13.0 min.
7.3.5 Validation of the analytical method and confirmation of the residue
Prior to extraction, series of control samples can be fortified with ethofenprox standard solution in acetonitrile at different concentrations. After standing for 2 h, analytical procedures mentioned above can be carried out to estimate the percent recoveries from different fortification levels.
7.4 Thiamethoxam and chlorantraniliprole residue analysis in rice grain (Adopted from Telo et al., 2015)
Thiamethoxam is a neonicotinoid systemic insecticide recommended against BPH and chlorantraniliprole is an anthranilicdiamide insecticide recommended against stem borer and leaf folder. Analytical method for thiamethoxam and chlorantraniliprole from rice hull, bran, or polished grains has been standardized separately.
7.4.1 Sample preparation for accelerated sample extraction (ASE)
Accelerated Sample Extraction (ASE) device is the latest special equipment helpful in efficiently extracting pesticide residues by utilizing small quantity of test samples and small quantity of the solvent through repeated extraction. Many a times, major quantity of the solvent can be obtained through condensation and reutilized. Thus it works out to be more economical also along with enhanced efficiency. The sample can consist of 2 g of rice hull, bran, or polished grains, 3 g of Chem tube hydromatrix, and 2 g of Florisil. Sample preparation can be carried out in a 50 mL beaker, and the contents can be mixed with a spatula to obtain a free-flowing powder. The cell size can be a 22 mL stainless steel cell, with two circular cellulose filters (size = 19.8 mm in diameter) at the low end of the cell. To estimate the percentage recoveries, Fortification of the samples can be done by adding appropriate volumes of solution to the samples, and equilibrating in the extraction cells at room temperature for 10 min.
7.4.2 Optimization of ASE conditions
Optimal extraction conditions standardized for obtaining highest chemical recoveries are; extraction temperatures of 75°C for rice hull and 100°C for rice bran and polished grains, pressure of 10.3 MPa, with acetonitrile (100%) solvent. The duration of static phase can be 5 min, following 1 min of preheating and 5 min of equilibration. The solvent can be collected in 60 mL vials with Teflon septa. Later on, each extraction cell containing the same sample can go through another identical extraction cycle, and the solvent can be collected in the same vial.
7.4.3 Optimization of evaporation conditions
The total extracted volume of 60 mL can be transferred into 15 mL volumetric tubes 10 mL at a time for sample evaporation using a Evaporator. TurboVap LV Evaporator is special equipment where small quantity of the samples is evaporated repeatedly at low temperature (45°C) to dryness using a gentle stream of nitrogen. The residue containing TurboVap LV Pesticides can be suspended in 2 mL of acetonitrile and shaken in a vortex mixer. The samples can be stored at 4°C during the 2 h period to complete the preparation of all samples and begin analyses with ultra-high pressure liquid chromatography (UHPLC).
7.4.4 Chromatography conditions for thiamethoxam and chlorantraniliprole residue analysis in rice grain
Analyses are performed using an UHPLC-MS/MS, model Acquity TQD. Chromatographic separation can be done with an ethylene bridged hybrid (BEH) C18 column (50 × 2.1 mm; 1.7 μm) with controlled temperature of 30°C and a 5 μL injection volume. Mobile phase is pumped from two solvent reservoirs consisting of 0.05% of formic acid in water (A) and acetonitrile (B) in an isocratic run (50 A/50 B) with a flow rate of 0.4 mL min-1, and the estimated void time for this method is 0.3 min. The mass spectrometer is operated using electrospray ionization (ESI) in positive mode, capillary voltage at 2.2 kV, source and dissolution temperatures of 150 and 400°C. Dissolution and cone gas flows are 800 and 50 L h-1, respectively. Nitrogen is used as dissolution and cone gas and argon (AOC) as collision gas. The pesticides analyzed are confirmed using selected reaction monitoring (SRM) mode in which ionizations of the parent compound and one product are analyzed and monitored for each compound studied.
7.4.5 Limit of detection (LOD) and limit of quantification (LOQ)
The quantification is based on a seven-point calibration curve (0.0005, 0.001, 0.002, 0.01, 0.1, 0.5, and 1.0 mg kg-1). A matrix effect can be obtained by comparing each response generated from calibration standards, which are prepared in pure acetonitrile and in matrix extracts. The limit of detection (LOD) is estimated by multiplying the response of the method noise level by approximately 3 and then converting the total response into an estimated concentration. The limit of quantification (LOQ) is estimated by multiplying the response of method noise level by 10 and then converting the total response into concentration of Thiamethoxam or chlorantraniliprole.
But the major lacuna in their study is that in rice like in other crops thiamethoxam is converted to clothianidin which is further metabolized to form N-demethylation of clothianidin to produce TZNG, hydrolysis of the nitroimino moiety to form N-(2-chlorothiazol-5-yl-methyl)-N’-methylurea (TZMU), denitrification to form N-(2-chlorothiazol-5-yl-methyl)-N’-methylguanidine (TMG), and cleavage of C-N bond within TMG to form methylguanidine (MG). Hence in the residue analysis for thiamethoxam also, clothianidin and its metabolites need to be considered and looked into.
7.4.6 Alternative method for chlorantraniliprole
Zhang et al., (2012) developed an alternative method for chlorantraniliprole analysis in brown rice and rice straw. Chlorantraniliprole residues can be extracted from samples with acetonitrile. The extract is cleaned up with QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) method, and determined by high-performance liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS). The average recoveries are 83.6-89.3% from rice straw, and 84.9-87.7% from brown rice. The relative standard deviation is less than 15%. The limits of detection (LODs) of chlorantraniliprole calculated as a sample concentration (S/N ratio of 3) are 0.15 μg kg (-1) for brown rice and rice straw. The final residues of chlorantraniliprole on brown rice are lower than maximum residue limit (MRL) of 0.02 mg kg (-1) after 14 d Pre-Harvest Interval (PHI). Based on their study Zhang et al., (2012) recommended a dosage of 150 mL a.i. of chlorantraniliprole/ha as safe to human beings and animals which is much higher than actual recommendation of 30 g a.i./ha for stem borer and leaf folder.
7.5 Imidacloprid residues in rice grain (adopted from Niaz et al., 2016)
Imidacloprid {1-(6 chloronicotinyl)-2-(nitroimino) imidazolidine} is a systemic insecticide which has been found to be highly effective against BPH and recommended in almost all Asian countries.
Niaz et al., (2016) Analyzed rice samples collected from different parts of Pakistan by using Waters HPLC 2,695. The system is equipped with Waters 2,996 photo diode array detector and computer with Empower software. The HPLC column KROMASIL 100 is used with C18 as stationary phase, 5 μm pore size and 250 x 4.6 mm dimension purchased from Teknokroma®.
7.5.1 Sample preparation for imidacloprid residues in rice grain
The QuEChERS method (Anastassiades et al., 2003) can be used with some modifications. Rice samples are ground to a fine homogenized powder using a standard food processor and homogenizer. Ten grams of powdered rice is weighed in centrifuge tube, followed by addition of 10 mL distilled water and 10 mL Acetonitrile (HPLC grade) mixed thoroughly with vortex. This is followed by addition of 4 g of MgSO4 and 1 g of NaCl. After vigorous shaking by hand for 1 minute, it is centrifuged for 5 minutes at 4,000 rpm. The extract is passed through activated solid phase extraction (SPE) cartridge. (In SPE a solid is utilized to remove extractives from the pesticide. This is more efficient than the liquid extraction and effectively reduces contamination to the analytical column and helps to obtain a linear base line of recorder attached to the detector.) After shaking the filtrate vigorously by hand for 1 minute it is centrifuged again for 3 minutes at 3,000 rpm. Finally, the extract is filtered with disposable nylon membrane filter (0.45 micron) and transferred into 2 mL vial for analysis at HPLC.
7.5.2 Insecticide standard solutions
The insecticide standard stock solution (1,000 μg mL-1) and intermediate standard solution (100 μg mL-1) can be prepared in acetonitrile. Intermediate standard solution is used immediately for getting the calibration curve and a linear equation. Blank samples with all glassware and reagents except the active ingredient can be periodically run for checking cross-contamination.
7.5.3 Chromatographic conditions
The mobile phase composition used is acetonitrile: water (65:35% v/v). The analysis is carried out in isocratic mode at a flow rate of 0.8 mL/min, with column effluent being monitored at 270 nm UV wavelength.
7.5.4 Method validation for imidacloprid residues in rice grain
The analysis method can be validated for parameters like linearity, accuracy, precision, limits of detection (LOD) and quantification (LOQ). Linearity can be determined by plotting resultant peak areas against different known concentrations (0.005, 0.01, 0.05, 0.1, 0.5, 1, 5 and 10 μg mL-1). The method accuracy and precision can be determined by recovery tests, with samples fortified at concentration levels of 0.01, 0.05, 0.1, 0.5 and 1 μg g-1. The limit of detection (LOD, μg mL-1) can be determined as the lowest concentration with a response of 3 times the baseline noise and limit of quantification (LOQ, μg mL-1) as 10 times the baseline noise. Imidacloprid residues in samples can be identified by matching the retention time with external standard. The concentrations in samples are calculated with the help of calibration curve.
7.6 Pymetrozine residues in rice grain (Adopted from Li et al., 2011; Zhang et al., 2015)
Pymetrozine is a novel insecticide that kills sap-sucking insects by entirely different mode of action not very clearly understood so far. However, the most recent report by Nesterov et al., (2015) clarifies the point about the mode of action of pymetrozine at cellular level. pymetrozine is observed to target transient receptor potential (TRP) ion channel complex that is unique to insect stretch receptor cells (Krishnaiah, 2017).
Pymetrozine residues from rice grain and rice straw can be extracted with acetonitrile, cleaned up by solid phase extraction (SPE) and then determined by high-performance liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS-MS). The average recovery ranges from 83.4-88.6% from rice straw, and 82.9-85.3% from brown rice. The relative standard deviation (RSD) is less than 15%. The limits of detection (LODs) of pymetrozine is 0.001 mg kg (-1) for brown rice and rice straw. At a dosage of 300-600 g a.i./ha, the highest final pymetrozine residues in brown rice are 0.01 mg/kg, which is lower than the European Union's upper limit of 0.02 mg kg (-1) in rice.
7.7 Clothianidin residues in rice grain (Adopted from Chen et al., 2005)
Clothianidin is a neonicotinoid insecticide which is extensively metabolized in rice as well as other crops. The major compounds of toxicological interest and need to be included in residue analysis are, the unaltered parent compound, TZNG formed due to N-demethylation of clothianidin, TZMU (N-(2-chlorothiazol-5-yl-methyl)-N’-methylurea) formed due to hydrolysis of the nitroimino moiety, TMG (N-(2-chlorothiazol-5-yl-methyl)-N’-methylguanidine) formed due to denitrification, and MG (methylguanidine) formed due to cleavage of C-N bond within TMG. However, MG is a naturally occurring compound and, as such, is not a residue of concern with regard to rice grain.
7.7.1 Extraction and cleanup for clothianidin residues in rice grain
A 5 g sample of unpolished rice can be transferred into a glass jar and homogenized for 1 min with 50 mL of methanol. The homogenate can then be filtered under reduced pressure through a funnel using Advantec No. 2 filter paper. The volume of filtrate can be raised to 200 mL by the addition of methanol. A 10 mL portion of sample can be evaporated to remove methanol under reduced pressure. The residue can be dissolved in 1 mL of 0.005 M 1-sodium octansulfonate (pH 8.3) and applied to an ENVI-CARB cartridge coupled with a HLB cartridge for solid phase extraction (SPE). (SPE is latest equipment where stationary solid material instead of traditional liquid, extracts the contents of the sample; hastens the process of clean up and reduces substantially the sample contaminant load on detection system). The combined cartridges can be previously conditioned with 5 mL of methanol, 3 mL of water, and 3 mL of 0.005 M 1-sodium octansulfonate (pH 8.3), followed by sample loading with 6 mL of 0.005 M 1-sodium octansulfonate (pH 8.3) twice, and washed with 3 mL of 1% acetonitrile in 0.005 M 1-sodium octansulfonate (pH 8.3). Clothianidin and its four metabolites can be eluted from cartridges using 3.5 mL of 70% acetonitrile in 0.005 M 1-sodium octansulfonate (pH 7). The eluent can be evaporated to 2.5 mL under nitrogen and reconstituted to 5 mL with 0.1% acetic acid for HPLC analysis. A recovery study can be carried out in triplicate by fortifying 10 μg standards into 5 g testing samples to achieve a 2 ppm concentration level. Blank tests without fortification standards can also be performed to determine the contribution of background factors.
7.7.2 High performance liquid chromatography for clothianidin residues in rice grain
HPLC analysis is conducted on an Agilent 1,050 system equipped with a variable wavelength detector (VWD) and an Agilent Aq (15 cm × 2.1 mm I.D.; particle size, 5 μm) column. All runs can be acquired and processed using the Agilent Chem Station software. Detector wavelength can be set at 269 nm for MNG and at 254 nm for clothianidin and the other three metabolites. Clothianidin and its metabolites are eluted from the LC column by mobile phase consisting of 20% acetonitrile and 80% 0.005 M 1-sodium octansulfonate (pH 7). For regenerating the LC column and maintaining the repeatability of retention times, a mobile phase consisting of 60% methanol, 30% acetonitrile, and 10% water can be employed to wash the column. Injection volume can be 50 μL. Standards can be injected between sample injections. Identification and quantification of clothianidin and its four metabolites in test samples can be accomplished by comparing the retention time and peak area of samples to those of the standards. The retention times of MNG, TZMU, TZNG, clothianidin, and TMG are 8.8, 13.7, 14.7, 15.7, and 16.7 min, respectively. Average recoveries of clothianidin, TZNG, TMG, TAMU, and MNG are 110%, 87%, 113%, 108%, and 61%, respectively. The limits of quantification (LOQ) are 0.05 ppm for clothianidin, TZNG, TZMU and MNG, and 0.1 ppm for TMG in rice.
7.8 Cyantraniliprole residues in rice grain (Adopted from Zhang et al., 2013)
Cyantraniliprole (3-bromo-N-[4-cyano-2-methyl-6-[(methylamino)-hydroxy]phenyl]-1-(3-chloro-pyridine-2-yl)-1-H-pyridine-5-formamide, DPX-HGW86) is an o-amino-benzamide insecticide in which a cyano group replaced the 4-halo substituent of the former anthranilic diamide chlorantraniliprole. The details about the mode of action of cyantraniliprole can be found elsewhere (Krishnaiah, 2017).
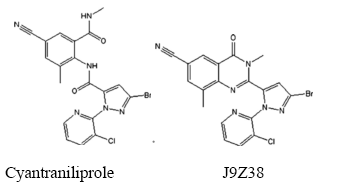
7.8.1 Extraction and cleanup for cyantraniliprole residues in rice grain
Approximately 10.0 g sample of dried brown rice can be weighed into a 150-mL conical flask. Acetonitrile (30 mL) is added. The mixtures are shaken vigorously for 1 h and then filtered. Then, the filtrate can be collected and fixed to 50 mL with acetonitrile. The solution (0.5 mL) obtained from the above 50 mL filtrate can be diluted with 0.5 mL deionized water and then mixed fully for purification. A silica gel column can be conditioned with 2 mL acetonitrile. The obtained solution (1 mL) can be loaded to the cartridge and collected in a flask. The analytes are eluted with 1 mL acetonitrile, and the eluate is collected. The eluate can be fixed to 2 mL with acetonitrile and filtered with 0.22 µm syringe filters for UPLC-MS/MS analysis.
7.8.2 UPLC system conditions for cyantraniliprole residues in rice grain
Chromatographic separation of cyantraniliprole and J9Z38 (a toxic metabolite of cyantraniliprole) can be performed on a Waters Acquity UPLC system comprising a Waters Acquity UPLC binary solvent manager, Acquity UPLC manager, and Acquity cartridge heater, equipped with a Waters Acquity UPLC BEH C18 column (2.1 mm 50 mm, 1.7 µm particle size). The mobile phase consists of solvent A (0.1 mM formic acid + 0.01 mM ammonium formate aqueous solution) and solvent B (acetonitrile 20:water 80, v/v). The mobile phase solvents are distilled and passed through a 0.22 µm pore size filter before use. The LC separation can be performed by injecting 2 µL at a flow rate of 0.20 mL min-1. The column can be kept at 40°C, and the temperature in the sample manager is set to 10°C.
Analysis of cyantraniliprole and J9Z38 can be conducted on a triple-quadrupole mass spectrometer (XEVO TQ MS, Waters, USA) using positive electrospray ionization (ESI+) mode. The capillary voltage and extractor voltage are set to 3.0 kV and 40 V, respectively. The source temperature and desolvation temperature can be held at 120°C and 400°C, respectively. The desolvation gas can be set to a flow rate of 800 L h-1. The collision gas, which was high purity argon, can be held at 0.15 mL min-1. All the parameters for multiple reaction monitoring transitions, cone voltage, and collision energy can be optimized to obtain the highest sensitivity and resolution. Under these conditions, the retention times of cyantraniliprole and J9Z38 are 0.89 and 1.09 min, respectively.
7.9 Fipronil residue analysis in rice grain
Kumar and Singh, (2013) attempted to analyze the residues of fipronil in rice plants and grain after granular application to soil at recommended and four times recommended dose of the insecticide. During the process they have standardized the methodology for analysis of fipronil in rice grain.
7.9.1 Sample collection extraction and clean-up for fipronil residue in rice grain
For quantification of fipronil residues from rice straw, about 2 kg of paddy straw can be randomly collected from each plot at the time of harvest. The samples are collected in labeled polyethylene bags and brought to the laboratory for analysis. Each sample is chopped, thoroughly mixed and reduced to appropriate sample size by successive sub-sampling. After harvesting and threshing, the entire amount of paddy seeds can be dried in sun for 2-3 days. From the paddy seed, husk and bran are removed with a rice huller in order to obtain polished rice grains and bran separately. Representative samples of rice straw, rice grain, bran and husk (20 g each), are dipped in 100 mL of acetone and kept overnight. The extracted samples can be re-extracted with 50 mL of acetone and filtered. Finally, a washing is given to the beaker with acetone to remove traces of residues of fipronil and its metabolites. The combined extract, thus obtained, can be transferred to a separatory funnel of 1litre capacity and diluted with 600 mL of 2 per cent aqueous solution of sodium chloride. To this, 100 mL of dichloromethane is added and shaken gently for 1 min. The contents are allowed to stand till a clear separation of two phases is obtained. The lower layer of dichloromethane is drained into a 500 mL beaker through one and half-inch layer of anhydrous sodium sulphate supported on a pre-washed glass wool in a funnel. The aqueous layer is re-extracted with 100 mL of dichloromethane. This is followed by extraction twice with 50 mL of hexane each time. The upper organic phase is passed through the same anhydrous sodium sulphate and combined with the contents already obtained. The sodium sulphate is washed with an additional 25 mL of dichloromethane. The combined extracts thus obtained are concentrated to 2-3 mL under vacuum in a rotary evaporator at a temperature below 35°C. The extracts are cleaned up by using silica gel as an adsorbent. Before use, silica gel is activated at 110°C for 2 h. A glass column (60 cm x 1.5 cm i.d.) can be packed with activated silica gel (10-12 g) mixed with 0.5 g of charcoal, in between the two small layers of anhydrous sodium sulphate supported on glass wool. The column can be pre-washed with hexane, following which the concentrated extract is poured over it. The glass beaker can be rinsed with acetone and the extract is transferred to the column. The extract can be eluted with a freshly prepared solvent mixture of dichloromethane and acetone (1:1, v/v). The eluate is concentrated to near dryness in a rotary evaporator under vacuum and transferred to 5 mL of acetone for further analysis.
7.9.2 Fipronil analysis with GLC with EC detector
The sample extracts can be analyzed using gas liquid chromatograph (GLC), model Shimadzu 2010, equipped with electron capture detector (ECD) and capillary column DB-5 (30 m x 0.25 mm i.d. x 0.25 µm film thickness of 5 per cent diphenyl 95 per cent dimethyl polysiloxane). The working conditions of GC are, injector temperature 280°C, column initial hold temperature 200°C for 5 min, followed by 220°C for 10 min and detector temperature 300°C. Carrier gas (N2) flow is maintained @ 30 mL min-1 with split ratio 1:10. Before use, the column can be primed with several injections of standard solution of fipronil till a consistent response is obtained. Suitable aliquots of the cleaned samples are then injected into GC with ECD mode of the detector. The compound in the sample is identified and quantified by comparison of the retention time and peak heights of the sample chromatograms with that of standard run under identical operating conditions.
7.9.3 Confirmation of fipronil residues by GCMS
The results can be confirmed by gas chromatograph mass spectrometer (GC-MS) in single ion monitoring mode. The gas chromatograph (GC) used is Shimadzu-QP 2010 equipped with mass spectrometer (MS) and a capillary column Rtx-5 Sil MS (30 m x 0.25 mm i.d. x 0.25 µm film thickness). The system software used is GC-MS solution version 2.5. The GC-MS operating conditions are: oven (program) initial temperature is 150°C and held for 2 min, ramped 10°C min-1 to 220°C and held for 5 min, then again ramped 1°C min-1 to 230°C, held for 10 min; injector temperature is 280°C, column temperature is 150°C and detector temperature is 290°C. Helium can be used as a carrier gas with a flow rate of 0.7 mL min-1.
The residues of fipronil and its metabolites are confirmed by using gas chromatograph mass spectrometer (GC-MS) (Figure1). The mass/ions ratio (m/z) and retention times (Rt) can be used for the confirmation of fipronil and its metabolites. The samples are injected and confirmed on electron ionization (EI) mode. The compounds can be identified based on m/z ratio of total ions chromatograph (TIC) and fragmentations of selective ions monitoring (SIM) compared with fragmentations of different mass number obtained with fipronil and its metabolites standards. The molecular mass of desulfinyl is 389.14 which can be confirmed by its ions dissociate into (m/z): 69, 179, 213, 281, 333, 369, 388 at (Rt) 11.80 min. Similarly, its other metabolite sulfide has molecular mass 421.10 confirmed by m/z: 69, 213, 228, 255, 351, 420 at Rt 15.80 min. The parent compound fipronil has molecular mass 437.1, confirmed by m/z: 351, 367 at Rt16.40 min. The sulfone has molecular mass 453.1, confirmed by m/z: 69, 213, 255, 355, 383 at Rt 19.70 min. The amide has molecular mass 455.1, confirmed by m/z: 44, 69, 255, 368, 385 at Rt 24.10 min (Kumar and Singh, 2013).
|
Figure 1 Structures of fipronil and its metabolites (fipronil amide, desulfinyl fipronil, fipronil sulfide, fipronil sulfone) |
7.10 Dinotefuran, ethiprole and sulfoxaflor residues in rice grain
Dinotefuran is the latest systemic neonicotinoid insecticide recommended against BPH. It has no cross resistance in BPH which has already developed resistance to other neonicotinoids like imidacloprid and thiamethoxam. The tolerance limit for dinotefuran in Rice grain is 9.0 mg/kg. There is no specific method for residue analysis of dinotefuran in rice grain in literature. Similarly no analytical methods could be seen in literature for ethiprole and sulfoxaflor the other insecticides recommended and used against BPH extensively in many Asian countries. However, Wang et al., (2012) established a method for determination of seven neonicotinoid insecticides residues in grains including brown rice, maize, millet and oat. This method involves using a dispersive solid-phase extraction and dispersive liquid-liquid micro-extraction cleanup followed by high performance liquid chromatography (HPLC). Based on an appraisal of the characteristics of HPLC, validation experiments can be conducted for seven neonicotinoid insecticides. In the method, dispersive solid-phase extraction involves using PSA and bonded C18 coupled with graphitized carbon black with acetonitrile as the eluted solvent. In the linear range of each pesticide, the correlation coefficient is R2 ≧ 0.99. At the low, medium and high three fortification levels of 0.05-0.8 mg kg-1, recoveries fell within 76-123%. The relative standard deviation is between 0.9% and 12.6% for seven neonicotinoid pesticides. Low limits of detection (0.002-0.005 mg kg-1) and quantification (0.007-0.018 mg kg-1) are readily achieved with this method for all tested neonicotinoid pesticides.
For analysis of organophosphates and carbamate insecticide residues in rice grain a lot of literature is available. Hence the information is omitted in the present paper.
8 Implications of Pesticide Residues in International Rice Trade
Every human being strives to reach his innate nature of Existence-Knowledge-Bliss (Sat-Chit-Ananda) or in other words everyone wants to lead a happy and enjoyable life. He is ready to move even to far-off countries in search of employment, business etc. that enable him to move from necessities to comforts and from comforts to luxuries. If we critically observe the world today it is like a small village with the advancements in civil aviation and information technology. There is large scale migration of people from Asian countries like China and India to advanced nations like USA and Canada and from South-East Asian countries like Thailand, Vietnam, Indonesia and Malaysia to fast developing Australia. Important point here is the migrants retain their original food habits and continue to cook rice in their homes. This necessitates large scale import of rice to USA, Canada and Australia from rice growing Asian countries like China, India, Thailand, Vietnam etc.
We have already seen in the earlier part of this paper that different organizations look into the aspect of pesticide residues in rice grain in all these countries. In many instances the tolerance limits or maximum residue limits for the same pesticide in rice grain may vary widely in the countries of origin to those in the destination countries. The values in the Table 1 amply illustrate this point.
|
Table 1 Tolerance limits or maximum residue limits of some pesticides (ppm) in rice grain in different countries revealing wide variation in many cases |
If we critically view the values in table we can make certain interesting observations. For azoxystrobin a fungicide widely used in rice there is 25 times variation between the values fixed by Japan to those of USA and Codex. In case of another fungicide tebuconazole a 30 times difference can be noticed in tolerance limits set in Japan to the value fixed by Codex. Similarly 10 times variation can be noticed in MRL values for Buprofezin one of the most important insecticides against BPH between Japan and India. With regard to dinotefuran another most important insecticide used against BPH the MRL value fixed in India is 0.03 ppm while the value fixed by codex (8.0 ppm) is 240 times higher and the tolerance limits fixed by Japan (2.0 ppm) and USA (2.8 ppm) are approximately 60 and 80 times higher. For Imidacloprid the MRL value set by Japan is 20 times higher than the value fixed by Codex and USA. The crux of the problem is when farmers use insecticides indiscriminately against BPH in rice exporting country like India, Thailand, China etc., and when the consignments reach in importing country like USA those are rejected. That creates lot of hardships to the exporters (Business Standard, New Delhi/Chandigarh September 17, 2012; Business Line on May 19, 2016). This speaks volumes about the importance of educating farmers about the right use of right pesticide on rice in general and against BPH in particular.
9 Conclusions
• The term of “pesticides” include insecticides, acaricides, fungicides, herbicides, rodenticides, nematicides, plant growth regulators, defoliants or desiccant, or the one used as a nitrogen stabilizer.
• In advanced countries like U.S. limited use of herbicides and some NPK fertilizers are permitted under organic cultivation while in countries like India there is total prohibition on pesticides and inorganic fertilizers under organic cultivation.
• Different agencies look after the issue of pesticide residues in food or feeds in different countries while “C O D E X A L I M E N T A R I U S” established by Food and Agricultural Organization (FAO) and World Health Organization (WHO) executes at global level.
• Similar to the development of pesticide technology the starting point for pesticide residue analysis is also DDT, HCH and the first generation organophosphate and methyl carbamates among insecticides and organo-mercurial and dithiocarbamates among fungicides, 2, 4-D and other related molecules among herbicides.
• Rice is a staple food for nearly 2.4 billion people in Asia. Except for Pakistan and some parts of India and China, rice provides two thirds of the calories for most Asians with rice-based diets. This reveals the importance of rice grain to be free from toxic contaminants.
• Fixing tolerance limit (TL) or maximum residue limit (MRL) for a pesticide is done after establishing acceptable daily intake (ADI) and maximum possible residues by following good agricultural practice (GAP).
• Because of peculiar chemical nature of buprofezin and nonamenability for GLC, there is need for developing residue analysis method by using HPLC. Extraction with acetone followed by partition with acetonitrile- hexane, florisil column chromatography and HPLC determination is employed.
• Ethofenprox being a simple hydrocarbon molecule with no atom or moiety sensitive to any specific detectors commonly used in GLC, there is need to develop HPLC method. The method for extraction, clean-up and determination of ethofenprox is almost similar to buprofezin.
• Thiamethoxam and chlorantraniliprole residue analysis in rice grain involve Accelerated Sample Extraction (ASE), TurboVap LV Evaporation followed by analysis with ultra-high pressure liquid chromatography (UHPLC).
• Alternatively, chlorantraniliprole residues can be extracted from samples with acetonitrile, cleaned up with QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) method, and determined by high-performance liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS). Imidacloprid Residues in Rice grain also employs almost similar methodology.
• Pymetrozine residues from rice grain can be extracted with acetonitrile, cleaned up by solid phase extraction (SPE) and then determined by high-performance liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS-MS).
• Clothianidin and its metabolites, TZNG, TZMU, TMG and MG can be extracted with methanol followed by solid phase extraction (SPE), High Performance Liquid Chromatography (HPLC) determination.
• Residue of Cyantraniliprole and its toxic metabolite (J9Z38) in rice grain is extracted with Acetonitrile, purified by silica gel column followed by UPLC-MS/MS analysis.
• Fipronil residues in rice grain are extracted with acetone; Cleanup can be carried out by partitioning with dichloromethane and hexane from aqueous phase followed by column chromatography with silica gel. Fipronil and its metabolites can be quantified by gas liquid chromatography and confirmed by gas chromatography mass spectrometer. For analysis of, dinotefuran a general method for many neonicotinoids is available; however, for ethiprole and sulfoxaflor residues in rice grain, no standard official procedures could be traced in literature.
• The crux of the problem is when farmers use insecticides indiscriminately against BPH in rice exporting country like India, Thailand, Vietnam, China etc., and when the consignments reach in importing country like USA, Canada, Australia or European countries those are rejected. That creates lot of hardships to the exporters which are reflected on to the rice farmers.
Authors’ contributions
The author along with his friends conceived the study participated in its design and coordination and drafted the manuscript.
Acknowledgments
The author is grateful to all the authors of original papers whose information is utilized for drafting. He is also thankful to his friends in Division of Entomology, Biotechnology and plant breeding, IIRR, Hyderabad, India for encouragement and help.
Anastassiades M., Lehotay S.J., Stajnbaher D., and Schenck F.J., 2003, Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce, Journal of Association of Official Analytical Chemists International, 86(2): 412-431
CAC (Codex Alimentarius Commission), 2003, Guidelines on good Laboratory practice in residue analysis, CAC/GL 40-1993, Rev. 1-2003, Rome, Italy
Casida J.E., and Durkin K.A., 2013, Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects, Annual Review of Entomology, 58: 99-117
https://doi.org/10.1146/annurev-ento-120811-153645
PMid:23317040
Chen M.F., Huang J.W., Wong S.S., and Li G.C., 2005, Analysis of Insecticide Clothianidin and Its Metabolites in Rice by Liquid Chromatography with a UV Detector, Journal of Food and Drug Analysis, 13(3): 279-283
FSASAI (Food Safety And Standards Authority Of India), Ministry of Health and Family Welfare, Government of India, New Delhi, 2015, Manual of Methods of Analysis of Foods, Pesticide Residues, Laboratory Manual 11, pp: 266
KFDA (Korea Food and Drug Administration), 2009, Analytical methods of pesticide residues in foods, In Korean code of food, pp: 10-4-10-10-4-18 and pp: 10-4-183-10-4-184
Krishnaiah N.V., 2017, Rice Brown Planthopper-A Global Scenario, M/S Sofia Publishers, Canada
Kumar R., and Singh B., 2013, Persistence and metabolism of Fipronil in rice (Oryza sativa Linnaeus) field, Bulletin of Environmental Contamination Toxicology, 90(4): 482-488
https://doi.org/10.1007/s00128-012-0926-y
PMid:23238826
Lee Y.D., and Jang S.W., 2010, Determination of Buprofezin Residues in Rice and Fruits Using HPLC with LC/MS Confirmation, Korean Journal of Environmental Agriculture, 29(3): 247-256
https://doi.org/10.5338/KJEA.2010.29.3.247
Lee Y.D., and Kwon C.H., 2004, Determination of monocrotophos residues in fruits and soils using high-performance liquid chromatography, Korean Journal of Environment and Agriculture, 23: 245-250
https://doi.org/10.5338/KJEA.2004.23.4.251
Lee Y.D., Kwon C.H. and Kwon K., 2011, Liquid Chromatographic Determination of Etofenprox Residues in Foods with Mass-Spectrometric Confirmation, Korean Journal of Environmental Agriculture, 30(4): 432-439
https://doi.org/10.5338/KJEA.2011.30.4.432
Li C., Yang T., Huangfu W., and Wu Y., 2011, Residues and dynamics of pymetrozine in rice field ecosystem, Chemosphere, 82(6): 901-904
https://doi.org/10.1016/j.chemosphere.2010.10.053
PMid:21074245
Nesterov A., Spalthoff C., Kandasamy R., Katana R., Rankl N.B., Andrés M., Jähde P., Dorsch J. A., Stam L.F., Braun F.J., Warren B., Salgado V.L., and Göpfert M.C., 2015, TRP Channels in Insect Stretch Receptors as Insecticide Targets, Neuron, 86(3): 665-671
https://doi.org/10.1016/j.neuron.2015.04.001
PMid:25950634
Niaz A., Sial R.A., Yaseen M., Mand G.A., Javed M.H., Ahmad E., Ahmad R., and Rahim M., 2016, Determination Of Imidacloprid Residues In Rice From Various Districts Of Punjab Using High Performance Liquid Chromatography, The Journal of Animal & Plant Sciences, 26(1): 170-176
PRARG (Pesticide Residue Analysis Research Group), 2006, Analytical methods of pesticide residues, pp: 128-130, 2nd edition, Chuohoki Publishing, Tokyo, Japan
RDA (Rural Development Administration), 2004, Test guidelines for pesticide persistence, In Criteria and Guidelines for Pesticide Registration, RDA Notification No. 2004-4, Annex 8, Korea
Teló G.M., Senseman S.A., Marchesan E., Camargo E.R., Jones T., and McCauley G., 2015, Residues of thiamethoxam and chlorantraniliprole in rice grain, Journal of Agriculture and Food Chemistry, 63(8): 2119-2126
https://doi.org/10.1021/jf5042504
PMid:25626153
Tomlin C.D.S., 2009, The Pesticide Manual, pp: 454-455, 15th ed., British Crop protection Council, Hampshire, UK
US EPA, 2010, Indexes to Part 180 Tolerance Information for Pesticide Chemicals in Food and Feed Commodities, Environmental Protection Agency 1200 Pennsylvania Avenue, N.W. Washington, DC 20460
US FDA, 1999, Pesticide analytical manual, Volume 1: Multi-residue methods, 3rd ed., US Food and Drug Administration, USA
Wang P., Yang X., Wang J. Cui J., Dong A.J., Zhao H.T., Zhang L.W., Wang Z.Y., Xu R.B., Li W.J., Zhang Y.C., Zhang H., and Jing J., 2012, Multi-residue method for determination of seven neonicotinoid insecticides in grains using dispersive solid-phase extraction and dispersive liquid-liquid micro-extraction by high performance liquid chromatography, Food Chemistry, 134(3): 1691-1698
https://doi.org/10.1016/j.foodchem.2012.03.103
PMid:25006000
Zhang C.Z., Zhang X.M., Tian Z.H., He D.J., and Liu X.J., 2010, Degradation of Chlorpyrifos and Fipronil in Rice from Farm to Fork and Risk Assessment, Agricultural Sciences in China, 9(5): 754-763
https://doi.org/10.1016/S1671-2927(09)60152-8
Zhang C., Hu X., Zhao H., Wu M., He H., Zhang C., Tang T., Ping L., and Li Z., 2013, Residues of cyantraniliprole and its metabolite J9Z38 in rice field ecosystem, Chemosphere, 93(1): 190-195
https://doi.org/10.1016/j.chemosphere.2013.05.033
PMid:23800585
Zhang J.M., Chai W.G., and Wu Y.L., 2012, Residues of chlorantraniliprole in rice field ecosystem, Chemosphere, 87(2): 132-136
https://doi.org/10.1016/j.chemosphere.2011.11.076
PMid:22205044
Zhang Y., Zhang L., Xu P., Li J., and Wang H., 2015, Dissipation and residue of pymetrozine in rice field ecosystem, Environmental Monitoring and Assessment, 187(3): 78
https://doi.org/10.1007/s10661-014-4256-x
PMid:25655126
. PDF(337KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Nagella Venkata Krishnaiah
Related articles
. BPH
. Pesticide residues
. Rice trade
. Analytical procedures
Tools
. Email to a friend
. Post a comment
.png)
.png)